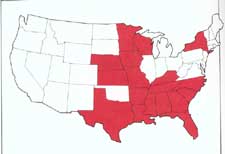
Brown Spot Needle BlightAlbert G. Kais - Principal Plant Pathologist (retired), Southern Forest Experiment Station, USDA Forest Service, Gulfport, MS. Cordell C.E., Anderson R.L., Hoffard W.H., Landis T.D., Smith R.S. Jr., Toko H.V., 1989. Forest Nursery Pests. USDA Forest Service, Agriculture Handbook No. 680, 184 pp. Hosts Brown spot needle blight, caused by the fungus Mycosphaerella dearnessii (syn. Scirrhia acicola), is common on longleaf pine seedlings within the natural range of longleaf pine, that is, within the Coastal Plain from North Carolina to Texas. The fungus also infects seedlings of slash, loblolly, and white pines in nurseries within or slightly beyond this area. Distribution
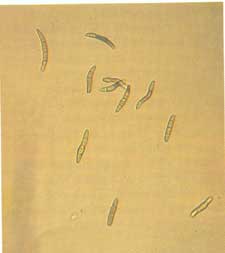
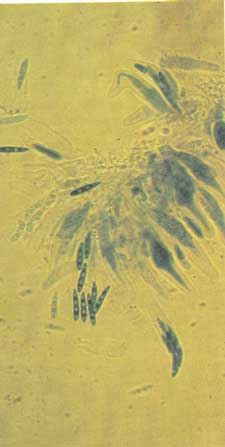
Diagnosis Although lesions may develop on secondary needles at any time, they most commonly appear from May to October. Look for small, grayish-green spots, which become a straw-yellow color and then light brown with chestnut-brown margins (fig. 1-2). Spots coalesce, and the needle tissue dies beyond and between groups of spots. Needles with multiple lesions appear mottIed. Infected needles have three distinct zones: a green basal portion, a mottled middle portion, and a dead apical portion (fig. 1-3). The fungus can also infect the cut ends of pruned longleaf pine seedlings. When this happens, entire beds may appear scorched (fig. 1-4). Two types of fruiting bodies are produced. Conidia are produced in acervuli, which appear on lesions as small black dots visible to the naked eye (fig. 1-5). Conidia are exuded in sticky masses up to 1mm long that split the epidermis of the leaf. These spores are cylindrical, curved, 1-4 septate, olive green to brown, and 19-35 x 3.5-4 microns (fig. 1-6). Ascospores are produced in pseudothecia embedded in dead leaf tissue. They are hyaline, oblong - cuneate, unequally two-celled, and 15-19 x 3.5-4.5 microns, with two prominent oil drops in each cell (fig. 1-7).
Conidia are produced in acervuli on the lesions resulting from ascospore infection. Conidia are disseminated short distances by rain splash and cause local buildup of the disease. Ascospores are produced on seedlings 2 to 3 months after the seedlings are infected. Both spore forms overwinter in dead and infected needle tissue. Control Prevention - Use superior seed that is resistant to the disease. Remove and destroy all infected seedlings and infected pines growing in and around the nursery. Cultural - In the spring, plant seed in rows at low densities of about 15 seedlings per square foot. Plant in well-drained beds. Use mulch to reduce mortality from sand splash, but do not use pine needles as mulch. Promote growth of existing ectomycorrhizal fungi by controlling soil pH (less than 6.0 is optimal) and avoiding excess levels of phosphorus. If necessary, inoculate beds with the mycorrhizal fungus Pisolithus tinctorius. Clip needles periodically during the growing season to prevent toppling and to expedite spraying and lifting. Remove clipped needles from nursery bed areas. Avoid pruning when it is raining or at any time when the seedlings are wet. Root prune seedlings to a depth of 7 inches from 6 to 12 weeks before lifting. Chemical - Spray with Bordeaux mixture, maneb, or chlorothalonil, which are effective and registered for use on brown spot. Seedlings should be sprayed wit a foliar fungicide at 10- to 30-day intervals - depending upon the amount of rainfall - from the beginning of April through October. Begin spraying in the spring when the new secondary needles are 1 to 2 inches long. Four to six applications are usually sufficient. Spray with a fungicide the day before pruning and again immediately after pruning. Swab cutting blades with denatured alcohol or a 10-percent solution of sodium hypochlorite. A seedling root-dip treatment in a 5-percent active ingredient benomyl-kaolin mixture prior to packing at the nursery or at the reforestation site is very effective in reducing brown spot in the field. This treatment is very economical and significantly improves both growth and survival of outplanted seedlings. Selected References Kais, AG. 1978. Pruning of longleaf pine seedlings in nurseries promotes brown spot needle blight. Tree Planters' Notes. 29(1): 3-4. Kais. AG.: Cordell. CE.; Affeltranger. CE.1986. Benomvl root t red t men I controls brown spot disease on longleaf pine in the Southern United States. Forest Science. 32: 506-511. Phelps. W.R.: Kais, A.G.; Nicholls, T.H. 1978. Brown spot needle blight of pines. For. Insect & Dis. 1.eall. 44. Washington, DC: U.S. Department of Agriculture. Forest Service. 8 p. |
Forest Pests: Insects, Diseases & Other Damage Agents |

|
|