Charcoal Root Rot and Black Root RotRichard S. Smith, Jr. - Plant Pathologist, Forest Insect and Disease Research Staff, USDA Forest Service, Washington, DC, Cordell C.E., Anderson R.L., Hoffard W.H., Landis T.D., Smith R.S. Jr., Toko H.V., 1989. Forest Nursery Pests. USDA Forest Service, Agriculture Handbook No. 680, 184 pp. Hosts Charcoal root rot, caused by the fungus Macrophomina phaseolina (syn. Sclerotium bataticola) affects more than 300 species of plants, including many agricultural crop plants, forest tree seedlings, and native weeds. All conifers are probably susceptible, but some appear to be much more so than others. In the West, the conifer seedlings most susceptible to charcoal root rot are true firs, Douglas-fir, giant sequoia, and sugar pine. Among southern conifers, slash, loblolly, longleaf, Virginia, sand, and south Florida slash pine seedlings are the most susceptible. A similar disease, black root rot, is caused by a complex of organisms of which M. phaseolina and Fusarium oxysporum are the most important components. Black root rot affects all species of southern pines grown in nurseries as well some hardwood seedlings such as sweetgum and dogwood.
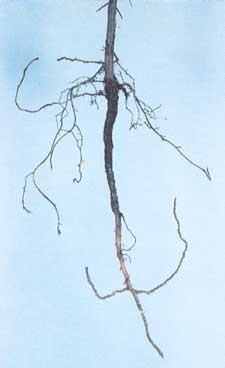
Diagnosis Symptoms of charcoal root rot are evident by midsummer and are characterized by a gradual decay of the root tips, lateral roots, and root crown. This gradual destruction of the root system causes the seedlings to become stunted and chlorotic, and finally to die (fig. 39-2). Symptoms of black root rot are similar, but can be distinguished by dark, roughened, enlarged areas on the taproot and larger laterals (fig. 39-3). Even though the tap-root remains alive, no regeneration occurs on the affected area, and often the root system consists of only a taproot stub. New roots may emerge above the affected area, but these too may become infected (fig. 39-4). Under normal regimes of fertilization and irrigation, foliage symptoms, such as chlorosis, wilting, stunting, and mortality, may or may not be evident. When water is withheld, seedlings with severely reduced root systems often die. Microsclerotia of M. phaseolina, readily visible with a 10 x hand lens, are frequently produced under the bark and epidermis on the lower stem and roots of seedlings affected by both diseases (fig. 39-5). Verification of Fusarium requires laboratory culturing of infected tissue. Macrophomina phaseolina also can be grown easily in culture, where it produces the characteristic black microsclerotia in large numbers. Biology Macrophomina phaseolina survives and overwinters as small, black microsclerotia in the soil and infested plant debris. When a growing root of a susceptible plant contacts a microsclerotium in the soil, this resting state germinates, and the fungus grows over the surface of the root and penetrates between epidermal cells into the root cortex. From there, the fungus advances through the cortex and inner bark into and up the taproot. The gradual destruction of the root system causes the seedlings to become stunted and chlorotic; finally they die. In the latter stages of decline and after the seedling has died, the fungus forms small, black microsclerotia in the inner bark of the roots and lower stem. As the stem and root system of the dead seedling decay and break down, these microsclerotia are released into the soil, where they serve as inoculum for next season's crop of seedlings. Fusarium oxysporum has a similar biology, except that it over-winters as chlamydospores. Both charcoal root rot and black root rot intensify during periods of high soil temperatures, low soil moisture, and excess nitrogen fertilization. Control Cultural - Maintain the organic matter in the soil at adequate levels (minimum of 2 percent) by planting cover crops and using other amendments to reduce disease occurrence and subsequent seedling damage. In selecting a nursery cover crop, favor nonhosts such as millet over known host cover crops, such as corn, peas, and sorghum. Avoid the application of high amounts of nitrogen fertilizers in nursery seedbeds where root rot is a problem. Rogue nursery seedbeds and cull lifted seedlings to remove and destroy all seedlings with potentially hazardous root rot disease. Disease cull standards should be established by the respective nurserymen and State pest quarantine agencies. Store, transport, and plant seedlings in ways that promote maximum seedling survival and growth and that minimize disease development and spread. Chemical - Before sowing seeds in nurseries where there is risk of root rot disease, fumigate seedbeds. The most effective soil fumigant is a formulation of methyl bromide and chloropicrin. Selected References Hodges, C.S. 1962. Black root rot of pine seedlings. Phytopathology. 53: 210 -219. Seymour. C.P.; Cordell. C.E. 1979. Control of charcoal root rot with methyl bromide in forest nurseries. Southern Journal of Applied Forestry. 3: 104-108. Smith, Richard S., Jr. 1975. Charcoal root disease In: Peterson. G. W.; Smith, Richard S., Jr., tech. cords. Forest nursery diseases in the United States. Agric Handb. 470. Washington. DC: U.S. Department of Agriculture: 11-13. Smith, R.S., Jr.; Bega, R.V. 1964. Macrophomina phaseolina in the forest nurseries of California. Plant Disease Reporter. 48: 206. |
Forest Pests: Insects, Diseases & Other Damage Agents |

|
|