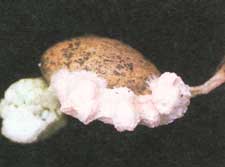
Seed FungiCordell C.E., Anderson R.L., Hoffard W.H., Landis T.D., Smith R.S. Jr., Toko H.V., 1989. Forest Nursery Pests. USDA Forest Service, Agriculture Handbook No. 680, 184 pp. Hosts Seed fungi, especially species of Aspergillus, Diplodia, Penicillium, Fusarium, Pestalotia, Trichoderma, and a number of Phycomycetes, affect the seed of all forest tree species. Distribution Seed fungi are found throughout the known ranges of all tree species. Some species of fungi, such as the pitch canker fungus, Fusarium subglutinans (syn. F. moniliforme var. subglutinans), appear to be confined to certain pine species, and the amount of damage they cause varies with geographic location. Damage The losses caused by seed fungi may occur during seed development, storage, or germination. Damage results from loss of seed viability or from seedling infection following germination. In general, however, losses associated with these fungi are low. Diagnosis Seed fungi are found on and in the seedcoat and in the gametophyte and embryo. With some exceptions, such as molds, these fungi cannot be detected by examining the outside of seeds. Some internal seed fungi can be detected by the visible presence of mycelium when the seed is cut open. However, the only accurate way to assess the incidence of seed fungi is to place samples of the seeds on appropriate culture media. After incubation, fungi can be detected by the presence of fruiting bodies (figs. 45-1 and 45-2) or mycelium (fig. 45-3) on the surface of the seed and the growth medium. Because of the large number of fungi that infect seed, a specialist is usually needed to identify them.
Biology A variety of fungi are found in association with the seeds of forest trees. Not all are pathogenic Some of these fungi, such as species of Diplodia and Fusarium, are pathogenic and may retard seed germination or cause damping-off, root rot, or other diseases of seedlings. Research has not pinpointed the mode of entry of internal seed fungi. They may enter during seed and cone development or through cracks in the seedcoat, especially after the seed has been extracted from the cone. External fungi could develop on the seed at any time after the seedcoat is formed. The types and amounts of seed fungi vary with the tree species, location, and year of collection. Fungus populations may increase during all phases of seed development and processing. It has been shown that seed collected from F. subglutinans-infected orchards frequently carries the same fungus. Seed-harvesting practices such as letting the seed fall on nets or the ground before harvesting seem to increase the incidence of seed fungi. Cone-processing procedures that result in high moisture and temperature conditions in the cone often increase seed fungi. Handling seeds during extraction can also attached. produce conditions favoring the development of seed fungi, in addition to damaging seed, which lets the fungus penetrate the seed. Fungi can also build up in storage if temperatures and seed moisture are not properly maintained. Cool, wet conditions at the time of seeding in the nursery seem to enhance these fungi and their subsequent damage. Control Prevention - Identify seed orchards that have seedlots with internal seed fungi. When possible, avoid using seedlots that are known to be infected with seed-borne pathogens. Identify seedlots that have a high occurrence of pathogenic fungi in unsound seeds and remove the unsound seeds. Avoid shipping seeds containing pathogenic fungi. Cultural - To detect possible disease development, monitor seedlots in a nursery known to contain pathogenic fungi. Seed Treatment - Several different types of direct seed treatment are available and have been used on certain tree species. Surface drying of several species of conifer seed has been effective in reducing some surface fungi. A 48-hour running water rinse reduces fungal and bacterial contamination on the seed of some pine species. Sterilization with laundry bleach reduces fungi on most tree seed. In general, these chemicals work better on the seeds with thicker coats; seeds with thin seed coats are more easily damaged. Captan, thiram, and benomyl have been reported to reduce seed fungi, but fungicide treatments are often selective in that they only affect one or two fungi and may reduce germination. Selected References Anderson, Robert L. 1986. Checklist of micro-organisms associated with tree seeds in the world, 1985. Gen. Tech. Rep. SE-39. Asheville, NC: U.S. Department of Agriculture. Forest Service. Southeast Forest Experiment Station. 34 p. Miller, Thomas; Bramlett, D.L. 1979.Damage to reproductive structures of slash pine by two seed-borne pathogens: Diplodia gossypina and Fusarium moniliforme var. sublglutinans. In: Bonner, frank, ed. Proceedings: flowering and seed development of trees: a symposium; 1978 May 5-6: Starkville. MS. New Orleans, L.A: U.S. Department of Agriculture, Forest Service, Southern Forest Experiment Station: 347-355. Sutherland, Jack H.; Miller, Thomas; Quinard, Rodolfo Salinas, eds. 1987. Cone and seed diseases of North American conifers. Publ. 1. Victoria. BC: North American Forestry Commission. 77 p. |
Forest Pests: Insects, Diseases & Other Damage Agents |

|
|